Abstract
Background
Waldenstrom macroglobulinemia (WM) is a rare haematological malignancy characterised by infiltration of the bone marrow by clonal lymphoplasmocytic cells and serum monoclonal IgM gammopathy. Rituximab based chemo-immunotherapy has long been the backbone of WM treatment. Bendamustine rituximab (BR), cyclophosphamide, dexamethasone rituximab (CRD) and bortezomib dexamethasone rituximab (BDR) are currently considered frontline options. The Bruton tyrosine kinase inhibitor (BTKi) ibrutinib in combination with rituximab (IR) has recently shown efficacy in treatment naïve WM and has been approved for use in this setting. With the expanding number of treatment options and absence of randomised trials (RCTs) comparing IR with chemo immunotherapy, there is uncertainty as to the optimal frontline treatment for WM.
Methods
The aim of this systematic review was to compare the efficacy and safety of rituximab-based chemoimmunotherapy combinations and IR, in treatment naïve WM. Using a pre-specified protocol in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA), MEDLINE, Embase, BIOSIS and Cochrane Library databases were searched for articles published from inception of each database to present. We included trials studying newly diagnosed WM patients treated with rituximab-based chemo-immunotherapy or BTKi based regimens. Primary outcome measures were progression free and overall survival (PFS and OS) while secondary outcomes included response rates and adverse events. A meta-analysis of proportions (expressed as a percentage), with their 95% CI were calculated.
Results
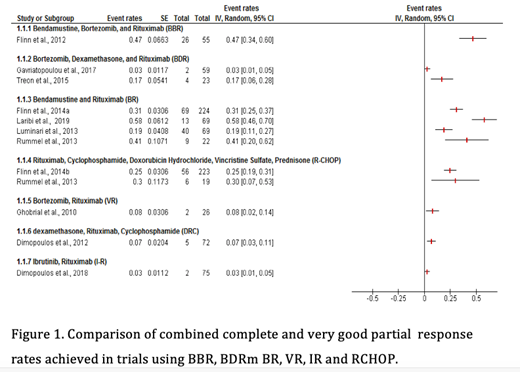
Of the 753 unique records identified, 8 single arm studies, one phase II non-randomised non inferiority study and 3 Phase III RCTs met the inclusion criteria. The sample size ranged from 23 to 261. PFS at 1- and 2-years were higher in BR(91%, 87%) and IR (93%, 82%), compared to BDR (87.5%, 66.8%) and CRD (82%, 64%) . Combined complete response (CR) and very good partial response (VGPR) rates were also superior with BR (35%) and IR ( 26%) compared to BDR (9%), Bortezomib- Rituximab (8% ) and CRD ( 7%) (Figure 1). The bortezomib bendamustine rituximab ( BBR) regimen was evaluated in only one study but showed an impressive CR/VGPR rate of 47% and a PFS at 3 years of 75%. Bleeding and atrial fibrillation were more common with IR, while neuropathy was more prevalent with bortezomib based regimens.
Conclusion
We present the first systematic review and meta-analysis evaluating frontline treatment options for WM in the BTKi era. Our findings suggest that bendamustine-based regimens may be superior in terms of depth of response and PFS. Longer follow up of the IR treated patients on the INNOVATE trial is however required to perform a definitive comparison of the regimens. The differential efficacy of these treatments in genomic subtypes of WM (based on MYD88 and CXCR4 mutational status) is an important question to be answered in future trials. While chemo-immunotherapy remains a potent, fixed duration option for newly diagnosed WM, BTKi based regimens are a valid alternative for patients who prefer an oral treatment or who are unfit for cytotoxic therapy. With the expanding number of treatment options for WM, RCTs comparing BTKi/immunotherapy with chemo-immunotherapy regimens are called for.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal